by Chris Riedel | Jun 10, 2023 | Compliance, Data, Uncategorized
Focusing on n-tier visibility on both sides of the OEM can mitigate disruptions, improve access to medical devices, and ultimately enhance patient care. In a recent Healthcare Technology Report article, the FDA is reported as once again raising concerns about medical...

by Brendan Sweeney | Apr 12, 2023 | Uncategorized
Since the pandemic, medical device sales has come under increasing pressure. Challenges have come from all sides, forcing many distributors and sales reps to adapt in order to overcome these challenges. Supply chain issues persist, making it difficult to ensure...

by Brendan Sweeney | Jul 14, 2022 | Data, Healthcare, Medical Device, Supply Chain, Uncategorized
You are, by now, probably familiar with the ongoing supply chain challenges brought to bear by recent global crises such as the COVID 19 pandemic and the Russian invasion of Ukraine. When the supply or flow of the supply is disrupted, management of current inventory...

by Brendan Sweeney | Jun 15, 2022 | Uncategorized
How are you managing your inventory? Do you even know where your inventory is? Do you know when inventory is going to expire, and do you have a plan for how to handle it when it does? It’s pretty common for us to hear that medical device companies, particularly new...
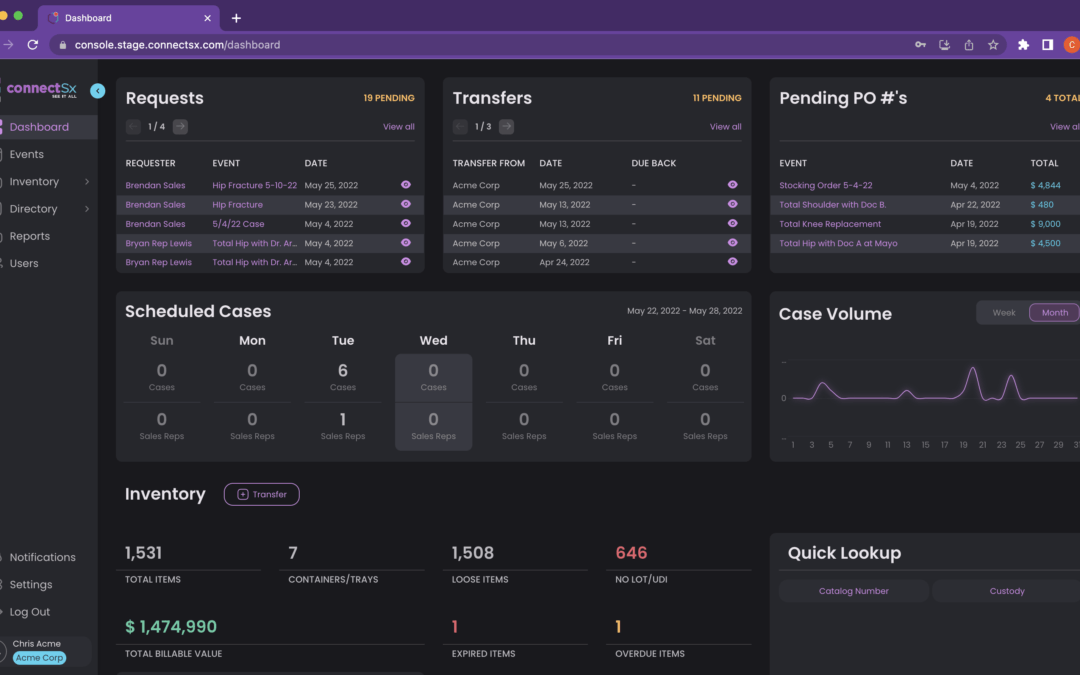
by Chris Riedel | May 28, 2022 | Uncategorized
We have recently launched a complete redesign of the ConnectSx Console. With refined navigation, a brand-new UI, and more intuitive workflows, this is our easiest-to-use version yet. With each update, we continue to learn from our customers and stakeholders,...

by Brendan Sweeney | Mar 11, 2022 | Uncategorized
Recently the FDA published a guidance requiring device manufacturers to be “Recall-Ready” noting, “It is critical that all companies in the supply chain are ‘recall ready’ to ensure appropriate actions are taken swiftly across the distribution channels to...






Recent Comments