Simplify the way you manage medical device inventory with UDidentify, a free app that makes it easy to scan, track and manage unique device identifiers.
The FDA now requires all medical devices to be labeled with unique device identifiers (UDI) that store important product details in GUDID, the global UDI database. By creating a standardized system for sharing critical information about medical devices, GUDID can advance the supply chain for manufacturers, distributors, sales reps and hospital systems – but only if your data is managed efficiently.
Is the burden of FDA UDI compliance leaving you buried in paperwork and inefficiencies? Or are you extracting value from each UDI label to drive visibility across the medical device supply chain?
The Advantages of Scanning with UDidentify
Simplify Device Tracking
Unlock FDA GUDID Data
Reduce Regulatory Stress
Enhance the Distribution Chain
Access These Unmatched Capabilities with UDidentify
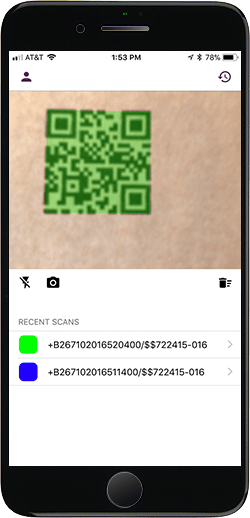
Simple UDI Scanning
Scan UDI label barcodes individually, or scan multiple barcodes on a packing sheet simultaneously, more quickly and accurately with UDidentify than with manual data entry.
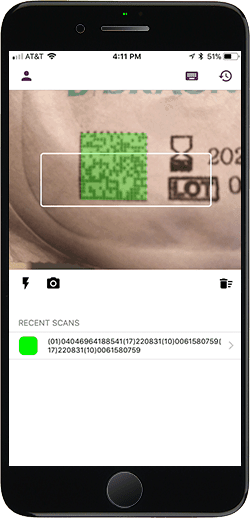

Effortless UDI Validation
Verify UDI barcodes in the global UDI database and validate them against HIBCC, GS1, and ICCBA standards. Who said UDI validation had to be difficult? UDidentify makes UDI easy.
Instant Device Info
Instantly access critical, comprehensive device data about devices from the GUDID database – including product details, expiration dates, lot numbers and manufacturer info.
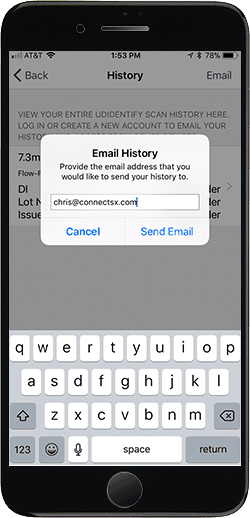

Easy Inventory Tracking
Track the history of all the medical devices you’ve scanned with UDidentify (manual intervention required for non-ConnectSx subscribers). Create a free account with your email address to send your detailed scan history to anyone.
Start Scanning UDI Labels the Easy Way
Want instant access to the GUDID database? Download UDidentify for free to simplify UDI label scanning and device tracking.
How UDidentify Stacks Up Against Other UDI Solutions
What makes UDidentify the best tool to manage device-specific data from the global UDI database?
More Accurate Than Manual Data Entry
More Convenient Than Additional Equipment
More Affordable Than Expensive Add-Ons
More Integrated Than Ordinary Scanners
Enhance Your UDI Tracking Efficiency
Start scanning UDI labels today for free to experience a more productive workflow tomorrow.



